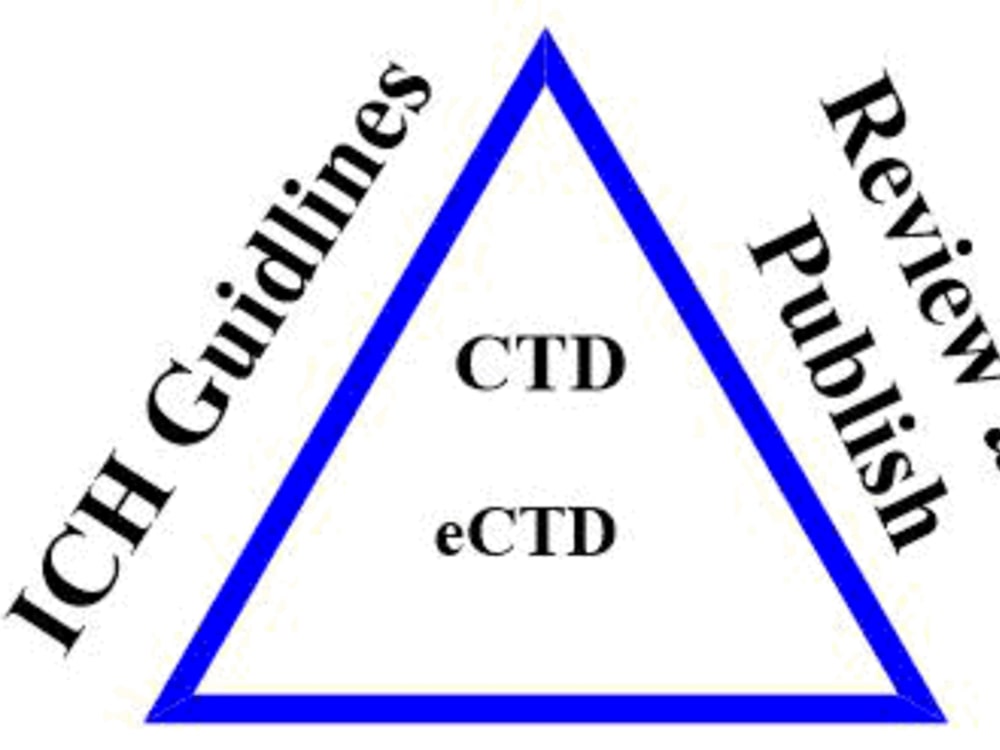
You will get stratey for the submission of drug product to FDA, EMA and MHRA
Top Rated
Project details
• Preparation of eCTD packages documentation per ICH and country specific guidelines for submission to FDA, EU, MHRA. Documents Required:
• Modules 1–5 of the dossier in Word or PDF per FDA and EMA pdf specification guidelines.
• Submission details: Country, MA number, company name, product name, API, etc.
• For existing eCTD lifecycles: Submission instructions
For regulatory affairs, CMC, or regulatory operations consultations, feel free to contact me.
• Modules 1–5 of the dossier in Word or PDF per FDA and EMA pdf specification guidelines.
• Submission details: Country, MA number, company name, product name, API, etc.
• For existing eCTD lifecycles: Submission instructions
For regulatory affairs, CMC, or regulatory operations consultations, feel free to contact me.
Industry
Physical SciencesLanguage
EnglishWhat's included
| Service Tiers |
Starter
$100
|
Standard
$200
|
Advanced
$1,000
|
|---|---|---|---|
| Delivery Time | 5 days | 10 days | 30 days |
Number of Revisions | 0 | 1 | 2 |
Frequently asked questions
5 reviews
(5)
(0)
(0)
(0)
(0)
This project doesn't have any reviews.
EO
Erin O.
Oct 9, 2025
Create OSHA/GHS Compliant SDS for Non-Flammable Hand Spray
YH
Yaniv H.
Aug 12, 2025
Registration with FDA, Label rev and registration
needed MoCra (FDA for cosmetics) help. A consultant wanted to charge us $2500 to do. We found zia for $200.. got the work done in half the time. Was far more knowledgeable and knew the process like back of his hand!
YL
YangYang L.
Mar 31, 2025
30 minute consultation
He is proficient and has in-depth knowledge of regulations and guidelines. He provided clear, agency-specific guidance, ensuring compliance with all requirements. Highly recommend his expertise!
SS
Stefanos S.
Oct 1, 2024
Bedrolite
Very good performance
JR
Jehan R.
Aug 11, 2024
FDA Regulatory Help
Fantastic work and getting all of our companies and products listed with the FDA. He clearly has in-depth knowledge and knows all regulatory requirements. He goes above and beyond and the communication with him is fantastic. Very professional and highly recommended
About Zia
Ph.D. Chemist | Regulatory | APIs | Drug Product | Cosmetic | Dietary
100%
Job Success
Malakand, Pakistan - 12:44 am local time
1. Drug Substance Regulatory Affairs
- Drug Master File (DMF) Writing
- API Supplier Sourcing
- ICH Q7 & cGMP Compliance
- Compliance Audits (API Manufacturing)
- Gap Analysis & RTR Prevention
- Global Market Approvals (API)
- End-to-End Regulatory Support
2. Drug Product Regulatory Affairs
- FDA Submissions (ANDA/NDA/IND)
- EU/MHRA Submissions (Hybrid Applications)
- eCTD Documentation (Modules 1-5)
- Gap Analysis & RTR Prevention
- End-to-End Regulatory Support
3. Cosmetic Product Regulatory Affairs
- FDA MOCRA Compliance and FDA Cosmetic Registration
- CPSR/PIF/SDS/COA Drafting
- EU/UK Cosmetic Notification or Registration
- Product Safety Assessments
4. Dietary Supplement Regulatory Affairs
- FDA Dietary Supplement Compliance
- Facility Registration
- Structure Function Claim Notification or Registration
- New Dietary Supplement
- FSVP Plan Guideline
🌟 Shared Across All Categories
🥇 100% Submission Acceptance
🥇 95% On-Time Delivery
🥇 60% Cost Savings
🥇 Free Consultation & Strategy
🔬 Regulatory Affairs Expert | Pharma & Cosmetic Compliance | 7+ Years | 100% Success Rate 🏆
With 7+ years of specialized expertise, I’ve empowered companies to secure
✅ Regulatory strategies (FDA, EU, MHRA) while saving clients 💸 $1M+ in costs through streamlined compliance and 🚫 zero Refuse-to-Receipt (RTR) risks.
✅ 32+ cosmetic registration approvals (cosmetic product, FDA, EU, UK). Created cosmetic product safety reports (CPSR), product information files (PIF), safety data sheets (SDS), and certificates of analysis (COA).
🚀 What I Deliver
1. 🧪 Drug Substance Expertise
Ensure seamless API compliance & DMF success:
✔️ DMF Writing & Review: Drafted 📑 30+ globally compliant Drug Master Files (USFDA, EU, ANVISA).
✔️ Gap Analysis: Fix deficiencies in existing DMFs to avoid regulatory delays.
✔️ 🔍 API Supplier Sourcing: Partner with 🌍 vetted global suppliers for high-quality APIs/excipients.
✔️ 🛠️ Compliance Audits: Conducted 12+ audits for API sites, ensuring ✅ 100% GMP adherence.
2. 📂 Drug Product Submissions (eCTD Modules 1-5)
From lab to market approval:
✔️ ANDA/NDA/IND Submissions: Delivered 📤 25+ ANDAs (FDA) + 15+ EU/MHRA hybrid applications.
✔️ 📊 eCTD Documentation: Expertly compile Modules 1-5 (CMC, clinical, non-clinical data).
✔️ 🚫 RTR Prevention: Zero Refuse-to-Review cases via meticulous gap analysis.
✔️ 📝 FDA Forms: Accurately complete 356h, 2253, and compliance declarations.
✔️ 🏭 Facility Registration: Register manufacturing sites per FDA/EU guidelines.
3. 💄 Cosmetic Compliance (FDA MOCRA/EU/UK)
Simplify global cosmetic market entry:
✔️ FDA MOCRA Compliance: Register firms/cosmetic products + Amazon Launch.
✔️ 🇪🇺 EU/UK Regulation: Register cosmetic products, prepare compliant Product Information Files (PIF), Safety Data Sheets (SDS), and Cosmetic Product Safety Reports (CPSR).
4. Dietary Supplement Compliance (FDA/EU/UK)
Simplify global dietary supplement market entry:
✔️ FDA DSHEA Compliance: Register firms/dietary supplements + Amazon Launch. Dietary Supplements Health and Education Act (DSHEA)
✔️ Structure Function Claim Notification or New Dietary Ingredient Notification (NDI)
🌟 Why Clients Choose Me
✅ Zero RTRs: 100% submission acceptance through rigorous gap analysis.
✅ ⏰ 95% On-Time Delivery: Met deadlines for 40+ projects in the last 3 years.
✅ 💰 60% Cost Savings
🛡️ My Ironclad Guarantee
✔️ Results-Driven: Work 🆓 FREE until you’re 100% satisfied.
ANDA submissions | eCTD publishing | FDA MOCRA compliance | Cosmetic registration FDA, EU and UK | CPSR, PIF, SDS and COA | DMF writing | API sourcing
✔️ 🔗 End-to-End Support: From API sourcing to final approval—I simplify every step.
📩 Let’s Launch Your Product Confidently!
👉 Next Steps:
Click 💬 “Send a Message” to discuss your regulatory needs.
Get a 🆓 FREE 15-minute consultation + tailored strategy.
Launch faster, smarter, and ✅ 100% compliant.
🏆 100% Success Rate | 🕒 7+ Years Expertise |
Your compliance partner for pharma & cosmetics—because every submission deserves to succeed. 🌟
Steps for completing your project
After purchasing the project, send requirements so Zia can start the project.
Delivery time starts when Zia receives requirements from you.
Zia works on your project following the steps below.
Revisions may occur after the delivery date.
Receive documents from client - Review Compile documents in eCTD formate